Xanthan
.........................................................................................
Introduction
Xanthan is the first bacterial polysaccharide produced by the strain Xanthomonas campestris and developed on a large industrial scale. This extracellular polysaccharide was first developed by Kelco Company. Xanthan has many industrial applications due to its suspending properties (stabilizer of solid suspensions or foams), its pseudo-plastic and thickening properties as a function of pH, temperature and salt concentration, and its ability to be cross-linked and gelled. Xanthan is mainly used as a stabiliser of emulsions, a thickener and a stabilizer of suspensions. It displays a relatively good stability during sterilization. It can be associated with of polysaccharides such as carrageenans, alginates, pectins, gelatin, galactomannans or glucomannans, for biomedical and pharmaceutical applications.
The primary structure is well established

 .
It consists of a pentasaccharide repeating unit. The backbone is cellulose like, i.e. made up of a linear β-D-(1→4) linked glucose residue. In its native form, every mannose residue attached to the backbone is acetylated. The terminal mannose has a variable degree of pyruvate substitution which depends upon the bacterial strain and fermentation conditions .
It consists of a pentasaccharide repeating unit. The backbone is cellulose like, i.e. made up of a linear β-D-(1→4) linked glucose residue. In its native form, every mannose residue attached to the backbone is acetylated. The terminal mannose has a variable degree of pyruvate substitution which depends upon the bacterial strain and fermentation conditions

 .
1H NMR allows one to quantify easily the yield in these two additional subsitutents (acetyl and pyruvyl). The presence of the glucuronic residue on the side chain confers to Xanthan an ionic character; the subsequent properties of the polyelectrolyte have been evaluated in great details. The conformation of xanthan in solution has been much described in the literature, and the debate is still whether a single or a double helix occurs in aqueous media. Data obtained by some researchers seem to indicate that xanthan is of a single chain conformation in the native state. However, thermal treatment above the the temperature of conformational change induces the formation of aggregates in dilute or semi-dilute aqueous solution. This causes difficulties in the interpretation of the physical behavior, more particularly from the rheological and light scattering experiments. .
1H NMR allows one to quantify easily the yield in these two additional subsitutents (acetyl and pyruvyl). The presence of the glucuronic residue on the side chain confers to Xanthan an ionic character; the subsequent properties of the polyelectrolyte have been evaluated in great details. The conformation of xanthan in solution has been much described in the literature, and the debate is still whether a single or a double helix occurs in aqueous media. Data obtained by some researchers seem to indicate that xanthan is of a single chain conformation in the native state. However, thermal treatment above the the temperature of conformational change induces the formation of aggregates in dilute or semi-dilute aqueous solution. This causes difficulties in the interpretation of the physical behavior, more particularly from the rheological and light scattering experiments.
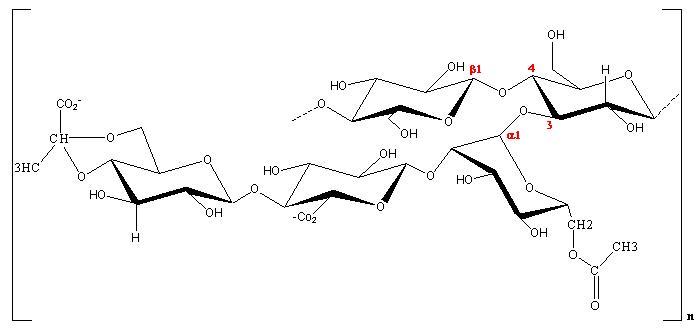
Fig. 1 Chemical repeat unit of Xanthan
X-ray diffraction patterns from xanthan show diffuse continuous intensities on layer lines and very few Bragg reflections

 .
Meridional diifraction spots on the 5n layer lines, along with the spacing suggest that the polymer has a 5-fold helix symmetry and a pitch of 47.0 Å. From the knowledge of the helical parameters alone, several speculative models have been proposed. They include single and double helices, parallel and anti-parallel chains, left- and right-handed chiralities, and up to four chains in the unit cells .
Meridional diifraction spots on the 5n layer lines, along with the spacing suggest that the polymer has a 5-fold helix symmetry and a pitch of 47.0 Å. From the knowledge of the helical parameters alone, several speculative models have been proposed. They include single and double helices, parallel and anti-parallel chains, left- and right-handed chiralities, and up to four chains in the unit cells

Fig. 2 Schematic representation of Xanthan chains
|








