Scleroglucan & Lentinan
.........................................................................................
Introduction
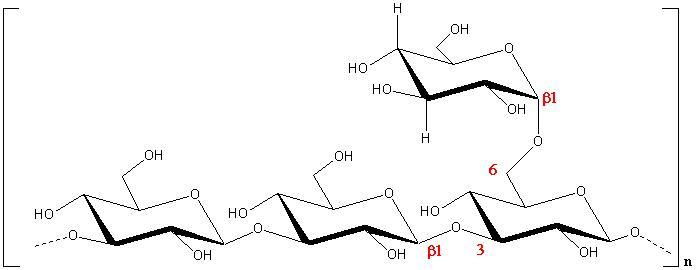
Fig. 1 Schematic representation of the repeating unit of Scleroglucan
These polysaccharides often form a triple helical conformation with a tendency to give a physical gel. Scleroglucan, lentinan, schizophyllan,…. all share similar structures and conformations. Their solubility increases in alkaline conditions, but an irreversible helix-coil transition occurs over pH > 12. Many of the polysaccharides belonging to this family are claimed to have anti-tumoral properties.
Scleroglucan & Lentinan
These β(1→3) / β(1→6)-glucans have been investigated mainly in relation to their biological activity, in addition to their applications as good thickening agents. Lentinan, is produced by the fungus Lentinus elodes. It has a β(1→3)-D-glucopyanosidic backbone with two β(1→6) glucopyranosidic branches every five glucose residues in the main chain. It has been approved as an anti-tumour agent against stomach cancer in Japan. Schizophyllan is produced by the fungus Schizophyllan commune and scleroglucan by Schizophyllan rolfsii or Botryosphaeria sp. They have the same backbone as lentinan but a single β(1→6)-D-glucopyranosidic unit as side chain.
Scleroglucan is the name given to a class of fungal polysaccharides secreted exocellularly by certain fungi of the genus Sclerothinia,. The polysaccharide produced by Sclerotium ghcanicum’ has been widely studied, but it is the exopolymer from Sclerorium rotfsii that is produced commercially at this time. Scleroglucan is a neutral polysaccharide. The optical rotary behaviour supports the concept (see below) that an ordered conformation exists in water and this is further corroborated by the absence of 13C-NMR signals for solutions in this solvent. The triple helical arrangement of scleroglucan has been established
 . .
|








