Glucomannans
.........................................................................................
Glucomannans
Glucomannan comprises up to 40% by dry weight of the roots of the bulbotuber of the Amorphophallus konjac a plant which cultivated in Asia. The polysaccharide is also present in large amounts in the wood of conifers and in smaller proportions in the wood of dicotyledons.
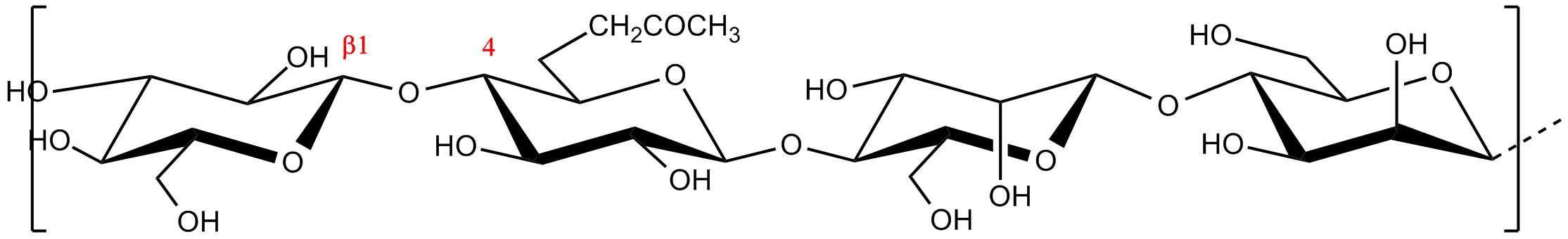
Fig.1 Schematic representation of the repeating unit of
The polysaccharide glucomanan consists of a linear arrangement of β-D-glucose (G) and β-D-mannose (M) residues linked by 1-4 linkages. There occur in an approximate ration of 5:8. The basic polymeric repeating unit has the following pattern; GGMMGMMMMMGGM. Short side chains of 11-16 monosaccharides occur at intervals of approximate 50-60 units of the main chain, attached by 1-3 linkages. Acetate groups at C-6 position occur at every 9-19 units along the linear chain. Hydrolysis of the acetate groups favours the formation of interchains hydrogen bonds, a feature which is partly responsible for the formation of gels and its subsequent high water absorption capacity and voluminous expansion.
A structural model of konjac glucomannan (mannose:glucose ratio 1.8) has been proposed based on X-ray data and stereo-chemical constraints
 . The unit cell dimensions of the orthorhombic I222 space group are
. The unit cell dimensions of the orthorhombic I222 space group are
a = 9.01, b = 16.73 and c (fiber axis) = 10.40Å. The structure crystallizes in the Mannan II polymorphic form in which the backbone conformation of a two-fold helix, and the chains are oriented in an antiparallel fashion.
Konjac glucomannan is water-solube diatary fiber which displays a wide range of applications, as a gelling agent, thickener, emulsifier and film former.
As a gelling agent, konjac glucoamnnan is quite unique in its ability to form thermo-reversible and thermo-irreversible gels under different and well controlled conditions. Solutions of native konjac mannan do not form gel because the acetyl content prevents the polysaccharide chains to approach and interact strongly. However, gels are obtained when the solution is heated at a pH of 9-10. These gels will remain stable to heat and under repeated heating up to 200°C. When exposed to slight alkaline environment, konjac solution forms thermo-irreversible gels after cooling from hot solution. The non-reversibility of the gel occurs from the removal of the acetate groups and partial structural crystallization occurs due to the formation of interchains hydrogen bonds. Making use of the non-reversibility property of konjac mannan has been the basis of a great variety of foods. Konjac mannan displays a synergistic effect with xanthan gum to form gels when used in a ratio of 3:2, as well as with other polysaccharides such as carrageenan, locust beam gum and starch.
Flour from the konjac plant is used traditionally to make Japanese shiritaki noods which are very low in calories. More recently, the glucomannan form the konjac plant has been exploited commercially as a thickening and gelling agent. It is used as a hunger suppressant because a feeling of fullness by creating viscous solution that delays absorption of the food nutrients. As dietary supplements glucomannans show healths benefits for constipation, cholesterol, diabetes and obesity. Because one gram of this soluble polysaccharide can absorb up to 200 ml of water konjac glucomannan is also used for absorbent articles such as disposable diapers and sanitary napkins
 .
.








