Gellan
.........................................................................................
Introduction
Gellan gum is a microbial polysaccharide that was discovered in 1978 in the laboratories of Kelco Division of Merck and Co. The micro-organism was isolated from the Elodea plant tissue, but subsequent investigations revealed that the gellan-gum producing bacterium was indeed a new strain of the species Pseudomonas, and thus called as Pseudomonas elodea. In 1994, it was discovered that the gellan producing bacterium was actually a member of the genus Sphingomonas and was called Sphingomonas elodea or Sphingomonas paucimobilis.
In its native form, the polymer is a linear anionic polysaccharide, made up of a tetrasaccharide repeating unit [→4)-l-rhamnopyranosyl-(α-(1→3)-D-glucopyranose-(β-1→4)-D-glucuronopyranosyl-(β-1→4)-D-glucopyranosyl-(β-1→) with O2 L-glyceryl and O6 acetyl substituents on the 3-linked glucopyranose residue.
a. 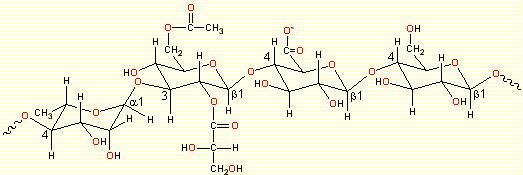
b. 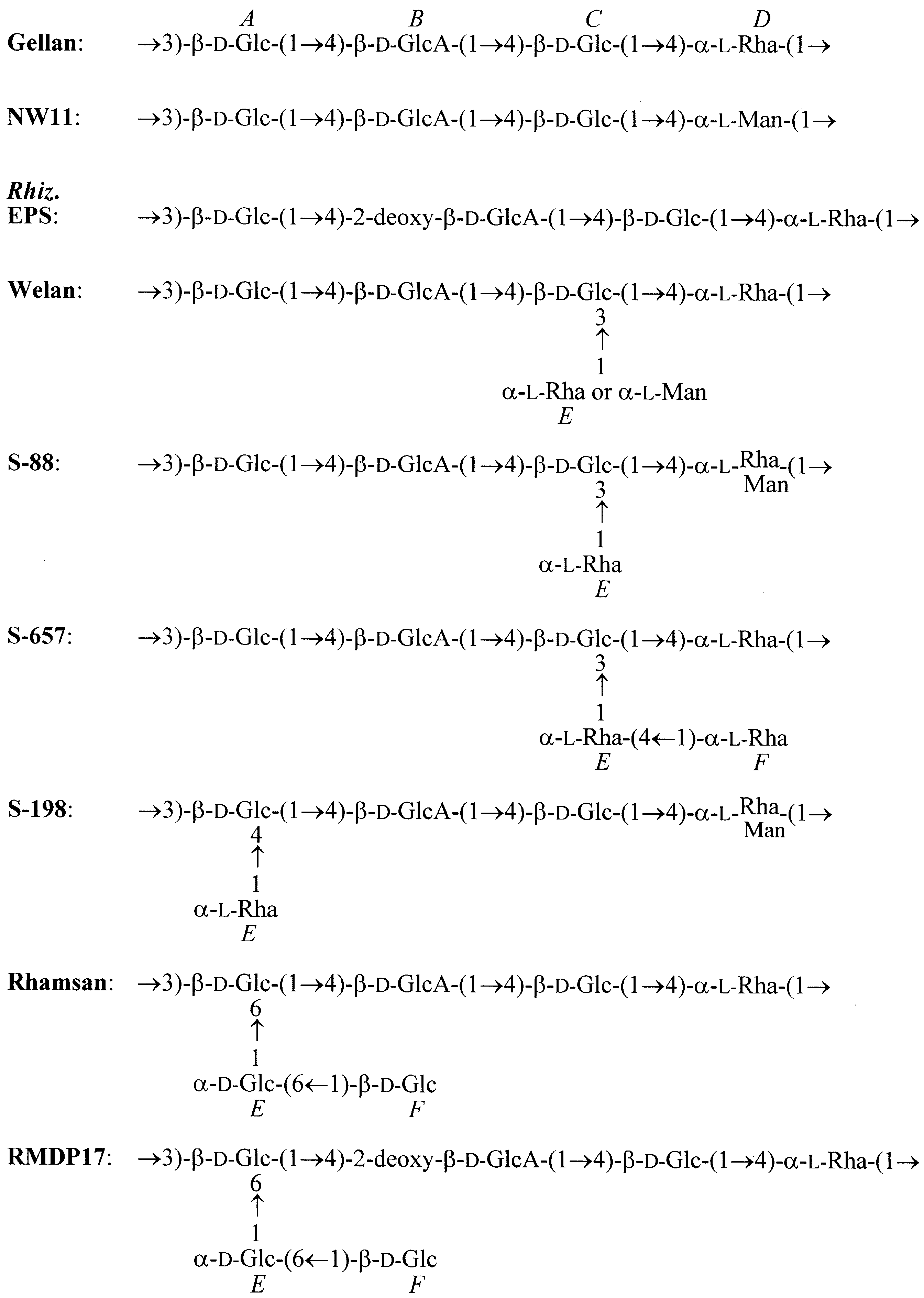
Fig. 1 Schematic representation of
(a) the repeating unit of Gellan
(b) comaprison of the primary structures of the Gellan family members
Acyl substituents drastically affect the rheology of the formed gels. Deacylation by alkali treatment results in a change from soft and more elastic thermoreversible gels to hard, firm and brittle gels.
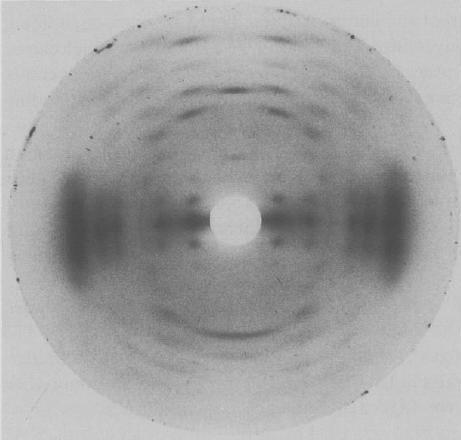
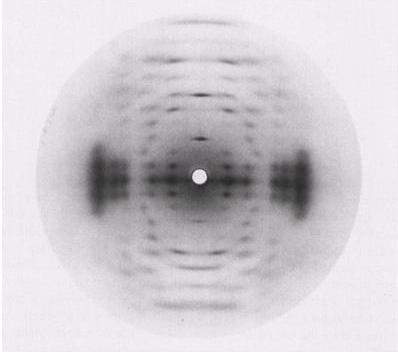
Fig. 2 X-ray fiber diffraction pattern of (a) the potassium salt of native Gellan (b) potassium salt of Gellan
Applications
Compared to other polysaccharides, gellan display important functional properties such as excellent thermal and acid stabilities, adjustable gel elasticity or rigidity, high transparency, and good flavor release  . Gellan gum received approval for use in food in Japan in 1988 and in the USA in 1992. Under the food additive code E418, it is uses as a natural bio-thickener, stabilizing and suspending agent
. Gellan gum received approval for use in food in Japan in 1988 and in the USA in 1992. Under the food additive code E418, it is uses as a natural bio-thickener, stabilizing and suspending agent  . Its applications are many fold: puddings, dessert gels, beverages, dairy products (yogurt, milk shakes, gelled milk and ice cream, fruit spreads, jams and marmalades, bakery filling, sauces
. Its applications are many fold: puddings, dessert gels, beverages, dairy products (yogurt, milk shakes, gelled milk and ice cream, fruit spreads, jams and marmalades, bakery filling, sauces  and
and  .
It is also used in fabricated foods, such as restructured meat, fruit and vegetables, and in canned/gelled pet foods. Due to its unique rheological properties, gellan gum is also exploited in non(food applications such as microbial and plant tissue culture media, solid matrix for DNA gel electrophoresis, the controlled release of certain drugs in the stomach, excipient for nasal and ocular drug delivery, material for the construction of scaffolds in tissue engineering, and in personal care products such as deodorant gels, sun screens, body lotions, hair conditioners and clear gel toothpaste
.
It is also used in fabricated foods, such as restructured meat, fruit and vegetables, and in canned/gelled pet foods. Due to its unique rheological properties, gellan gum is also exploited in non(food applications such as microbial and plant tissue culture media, solid matrix for DNA gel electrophoresis, the controlled release of certain drugs in the stomach, excipient for nasal and ocular drug delivery, material for the construction of scaffolds in tissue engineering, and in personal care products such as deodorant gels, sun screens, body lotions, hair conditioners and clear gel toothpaste 
 . Gellan is also used in the degradation of gasoline or for the encapsulation of bacteria for bio-augmentation of contaminated aquifers
. Gellan is also used in the degradation of gasoline or for the encapsulation of bacteria for bio-augmentation of contaminated aquifers 
 . The gellation properties and applications of gellans have been reviewed
. The gellation properties and applications of gellans have been reviewed
 .
.
The metabolic pathway of gellan is a multi-step process that initiates with the intracellular formation of the nucleotide sugar-precursors, followed by the formation of the repeating unit and ultimately, gellan polymerization and export 
 .
.
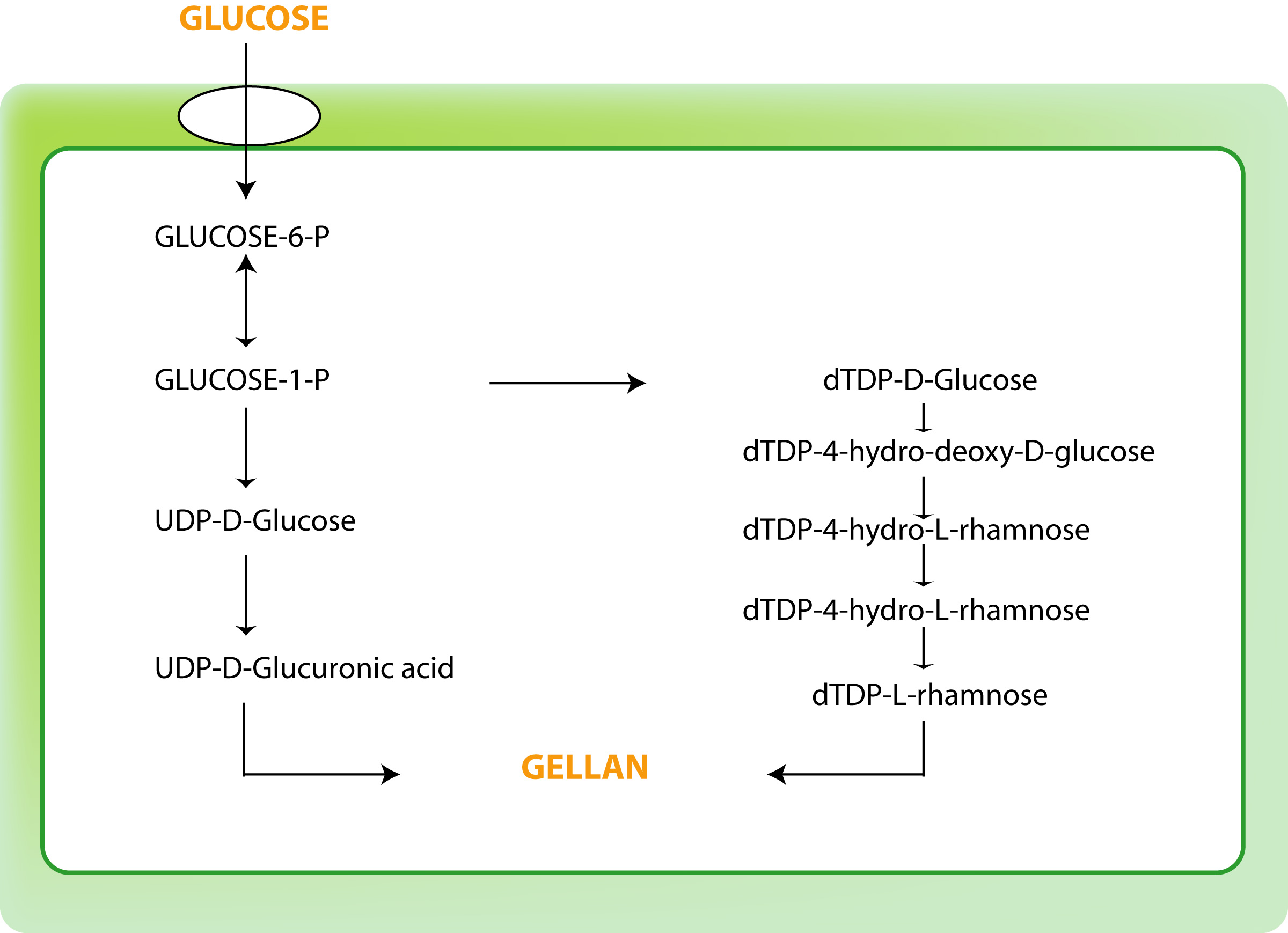
Fig. 3 The pathway of gellan synthesis
The native polysaccharide is produced by Pseudomonas elodea, and contains about 2-O-glyceryl and 6-O-acetyl substituents on one residue of the tetrasaccharide repeating unit. Removal of these substituents by alkali treatment yields a modified polysaccharide which has been referred to as gellan. The gellation properties of gellan depend on the amount and the nature of cation used.
The structure of lithium gellan was investigated from the diffraction pattern obtained from polycrystalline and well oriented fibers  .
The structure was established as a 3-fold left handed parallel, half staggered, double helix having a pitch of 56.4 Å.
.
The structure was established as a 3-fold left handed parallel, half staggered, double helix having a pitch of 56.4 Å.
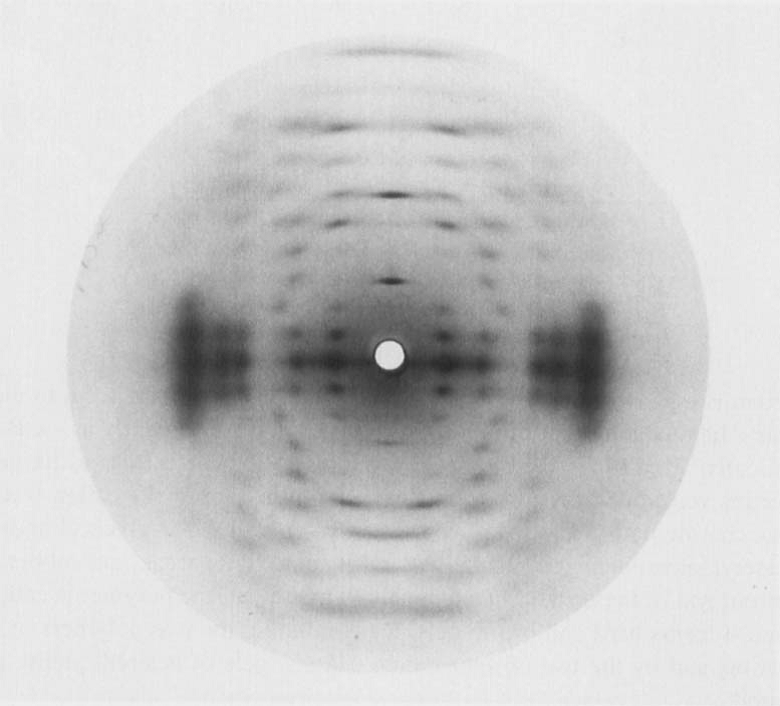
Fig. 4 X-ray diffractogram of of the potassium salt of gellan.
Further structural features were derived from other diffraction patterns of gellan complexed with other ions that yielded isomorphic structures. In particular, the quality of the diffraction pattern obtained with potassium provided enough experimental data to establish detailed molecular information. The axial rise per tetrasaccharide repeat is 18.77 Å. which corresponds to the maximum extension available, providing that the α-l rhamnose residue occurs in the 4C1 conformation whereas the three other monosaccharides take up the 4C1 conformation. With the space group symmetry being trigonal (P31) this implicates that the gellan chains arrange into a 3-fold double helical structure. The double helix is stabilized by both intra- and inter-chain hydrogen bonds.
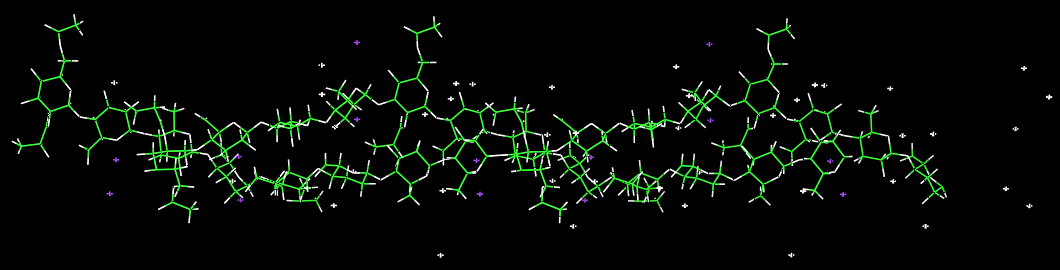
Fig. 5 Double helical structure of gellan and its potassium salt that incorporates the potassium ions (in its octahedral coordination) and the water molecules.
Within the unit cell, the double helices, which are separated by a lateral distance of 9.1 Å. are oriented in an antiparallel fashion.
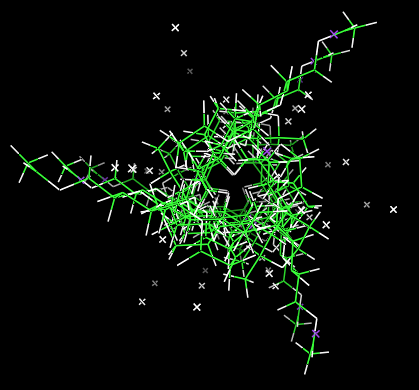
Fig. 6 Packing viewed perpendicular to the chain orientation
These experimental findings have been rationalized throughout computer modeling that indicated that the occurrence of a stronger inter-double helices interaction with calcium ions
 .
.
The investigation of the fiber diffractogram obtained for potassium native gellan
 revealed the occurrence of minor conformational changes resulting from 2-O-glycreyl and 6-0acetyl substituents. These changes do not alter the double helical structural arrangement but influence strongly the inter-double helical interactions, in a way such that many of the non-bonded and ionic interactions seen in the previous structures are no longer sustainable. This finding provides some structural basis to explain the functional differences between gellan and native gellan.
revealed the occurrence of minor conformational changes resulting from 2-O-glycreyl and 6-0acetyl substituents. These changes do not alter the double helical structural arrangement but influence strongly the inter-double helical interactions, in a way such that many of the non-bonded and ionic interactions seen in the previous structures are no longer sustainable. This finding provides some structural basis to explain the functional differences between gellan and native gellan.








