Dextran
.........................................................................................
Introduction
Dextran is a generic term used to describe D-glucans that contains a substantial numebr of (1→6)-linked-α-D-glucopyranopsyl residues. The great majority of dextrans are produced by bacteria growing on sucrose as a substrate. This kind of biological macromolecule is not crystallizable because of the occurrence of many branches. In contrast, linear dextran can be chemically synthesized. It has been found 
 that such a linear dextran can be crystallized from dilute solution in the form of lamellar single crystals, providing that low molecular weight and monodispersed fractions are used. Dextran, as many other polysaccharides exhibits polymorphism. A “low-temperature polymorph obtained below 100°C and a “high-temperature” polymorph crystallized above 120°C. The principle difference between these polymorphs is that the high-temperature polymorph is anhydrous while the low temperature crystalline form is hydrated. For both polymorphs, the crystal structure was established through a combine electron and X-ray diffraction analysis and stereochemical model refinement.
that such a linear dextran can be crystallized from dilute solution in the form of lamellar single crystals, providing that low molecular weight and monodispersed fractions are used. Dextran, as many other polysaccharides exhibits polymorphism. A “low-temperature polymorph obtained below 100°C and a “high-temperature” polymorph crystallized above 120°C. The principle difference between these polymorphs is that the high-temperature polymorph is anhydrous while the low temperature crystalline form is hydrated. For both polymorphs, the crystal structure was established through a combine electron and X-ray diffraction analysis and stereochemical model refinement.
The asymmetric unit of the anhydrous form is made-up of two non-equivalent glucose residues linked α (1→6)  . The conformation of the chain is relatively extended and ribbon-like, with successive residues in a near two fold screw relationship.
. The conformation of the chain is relatively extended and ribbon-like, with successive residues in a near two fold screw relationship.
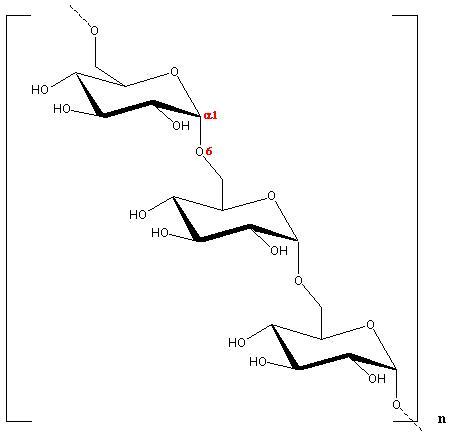
Fig. 1 Diagrammatic representation of the repeating unit of Dextran
a 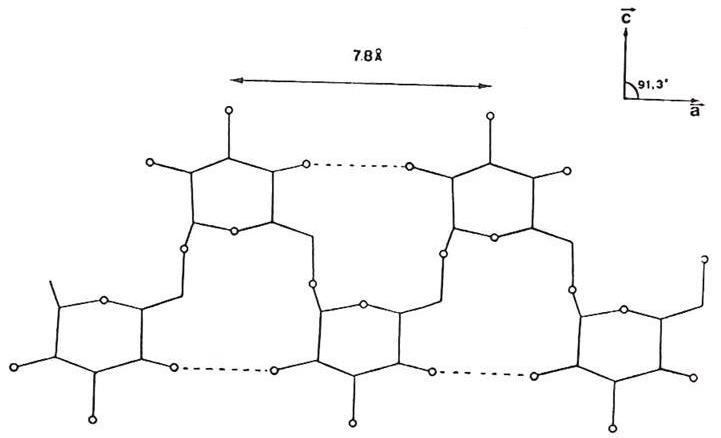
b 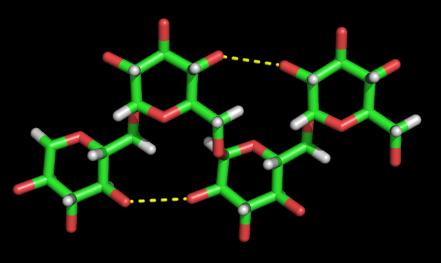
Fig. 2 Hydrogen-bonding seen in Dextran
(a) Diagrammatic representation
(b) Three dimensional representation
Two anti-parallel packed chains pass through the unit cell. The chains of like polarity pack into sheets with extensive intrasheet hydrogen bonding. The sheets packed anti-parallel, are in turn, extensively bonded together by intrasheet hydrogen bonding  .
.
a 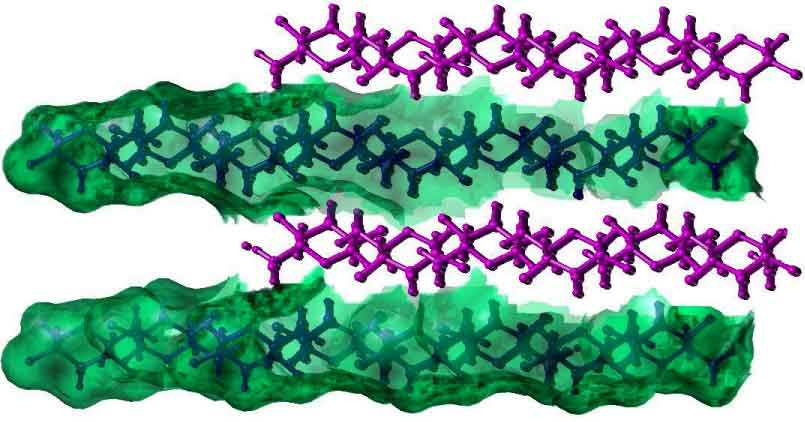
b 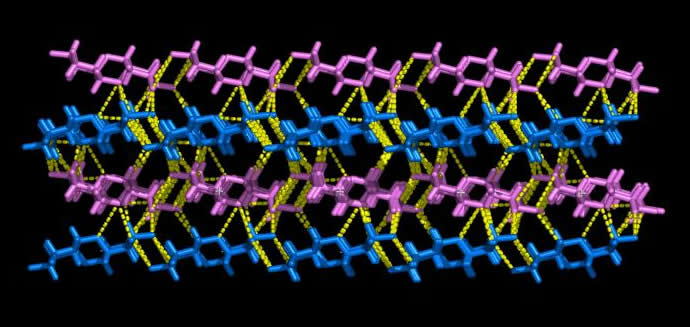
Fig. 3 Three-dimensional representation of Dextran chains
(a) Representation of the water accesible area (Connoly surface) in Dextran chains
(b) The Hydrogen bonding observed in the anti-parralel chains in a Dextran unit cell
The unit cell of the low temperature, hydrated dextran polymorph contains six chains and eight water molecules, with three chains of the same polarity and four water molecules constituting the asymmetric unit  . As in the anhydrous form, the chain is in an extended conformation, and close to a perfect twofold screw symmetry. However, the chain packing is different in the two structures, in that rotational positions of the chains about helix axis are considerably different. In the hydrated form, the chain packing is still quite tight, as is evident from intermolecular contacts, numerous hydrogen bonds and the high crystalline density. Water molecules occupy positions between sheets of chains, and play an essential role in hydrogen bonding. The water molecules of the asymmetric unit participate into 18 hydrogen bonds. There is no hydrogen bonding between these water molecules. The fact that these water molecules are tightly held in the crystal lattice explains the experimental observation that crystal of dextran hydrate do not deteriorate rapidly in the electron beam of the microscope. This is also reflected by the unusually high density of 1.68 of the crystalline samples
. As in the anhydrous form, the chain is in an extended conformation, and close to a perfect twofold screw symmetry. However, the chain packing is different in the two structures, in that rotational positions of the chains about helix axis are considerably different. In the hydrated form, the chain packing is still quite tight, as is evident from intermolecular contacts, numerous hydrogen bonds and the high crystalline density. Water molecules occupy positions between sheets of chains, and play an essential role in hydrogen bonding. The water molecules of the asymmetric unit participate into 18 hydrogen bonds. There is no hydrogen bonding between these water molecules. The fact that these water molecules are tightly held in the crystal lattice explains the experimental observation that crystal of dextran hydrate do not deteriorate rapidly in the electron beam of the microscope. This is also reflected by the unusually high density of 1.68 of the crystalline samples  .
.
Hydration of the dextran structure does not cause significant alterations in the conformation of the chains; it simply separates them. The distances between neighbouring chains of the same polarity along one axis of the unit cell are similar (9.2 Å in the anhydrous, 8.5 Å in the hydrate). However, the setting angles differ by approximately 60°. In both structures, the dextran chains form sheets. In the hydrate form, water separate sheets.
a 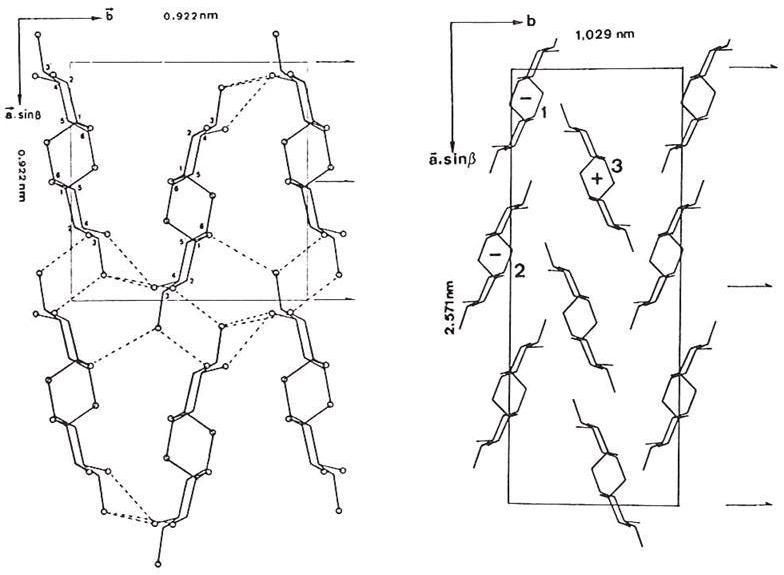
b 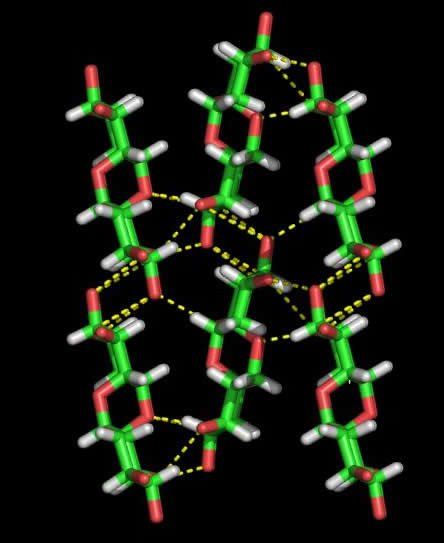
Fig. 4 Comparison of the two chain packings as seen in Dextran
(a) 2D Representation
(b) 3D Representation








