Carrageenans
.........................................................................................
Introduction
Carrageenans belong to a family of gel forming sulfated polysaccharides found in the marine red algae Rhodophyceae. Several members referred to as ι-, κ-, λ- carrageenans difer chemically in their degree of sulfation as well as in the position of the sulfate on the carbohydrate ring.
(a.) 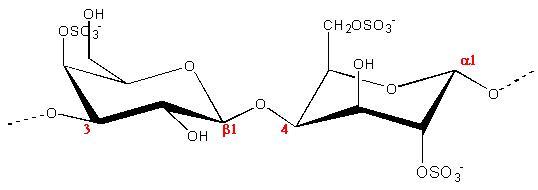
(b.) 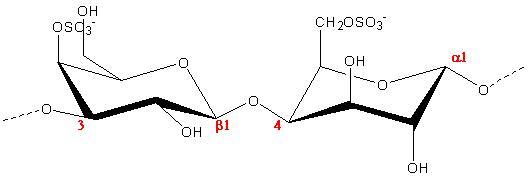
(c.) 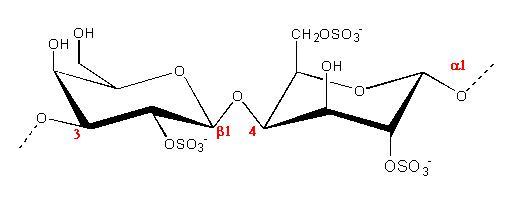
Fig.1 Chemical repeat unit of Carrageenans (a) Iota (b) kappa (c) lambda
As the molecular structures are not the same, significant functional properties are offered by this family of polysaccharides.
Kappa carrageenan binds water to form strong, rigid gels. Potassium salts are essential in order to form this firm gel structure. As the level of potassium is increased, the resulting gel structure becomes tightly aggregated and may cause syneresis (moisture on the gel surface).
Iota carrageenan also binds water, but forms a dry, elastic gel, especially in the presence of calcium salts. The divalent calcium ions help form bonds between the carrageenan molecules to form helices. The 2-sulfate group on the outside of the iota carrageenan molecule does not allow the helices to aggregate to the same extent as kappa carrageenan, but forms additional bonds through calcium interactions. The gels are more elastic, dry and provide excellent freeze/thaw stability.
Lambda carrageenan
Lambda carrageenan is highly sulfated and therefore less likely to form a gel structure. The ester sulfate distribution of lambda carrageenan is randomly distributed on the molecule. This prevents gelation and promotes viscous solutions. Lambda carrageenan is primarily used to thicken liquids and modify the texture of foods.
Iota carrageenan
Well oriented fibers of Ca2+, ι-carrageenan yield an X-ray diffraction pattern that indicate from the pitch (2c = 26.56 Å) and the presence of meridional reflections on the third and six layer lines, that the polymer conformation is a three-fold, right-handed, parallel, half-staggered double helix
 .
This model is only possible under the assumption of the occurrence of the 4C1 and 1C4 chair conformations in residue A and B, respectively. The glycosidic torsion angles corresponds to low energy domains of the corresponding disaccharides. The polysaccharide crystallizes in a trigonal unit cell that contains only one double helix.
.
This model is only possible under the assumption of the occurrence of the 4C1 and 1C4 chair conformations in residue A and B, respectively. The glycosidic torsion angles corresponds to low energy domains of the corresponding disaccharides. The polysaccharide crystallizes in a trigonal unit cell that contains only one double helix.
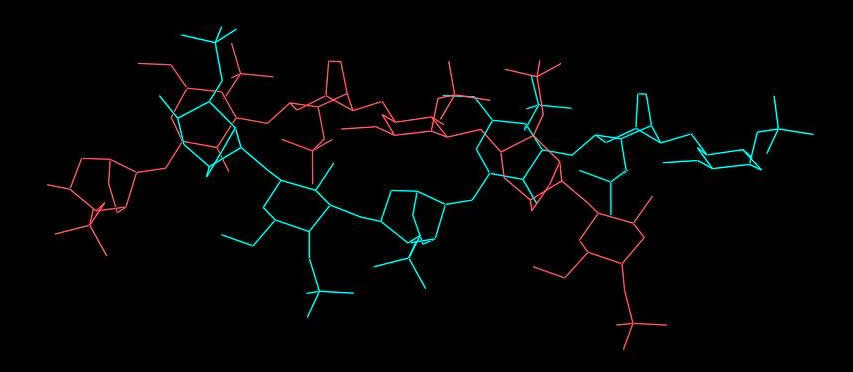
Fig. 2 Schematic representation of i-Carrageenan
A model of the packing arrangement has been proposed that incorporates a random distribution of up- and down- pointing double helices, distributed randomly at each cell corner. This would explain the presence of both Bragg reflections and layer lines streaks on the fiber diffraction pattern. Inter-double helix associations occur throughout SO4-….Ca2+…SO4- interactions, which form the basis of the moderate aggregation property of i-carrageenan compared to the k-form.
Kappa carrageenan
Fibers prepared from the potassium salt of k-carrageenan gives an X-ray pattern that shows only continuous intensities on layer lines, reflecting a low level of crystallinity and orientations
 .
The first layer-line spacing is 25 Å By analogy with the 3D structure of i-carrageenan, along with the occurrence of some similarities in overall intensity distribution, a double helical model has been proposed for k-carrageenans.
.
The first layer-line spacing is 25 Å By analogy with the 3D structure of i-carrageenan, along with the occurrence of some similarities in overall intensity distribution, a double helical model has been proposed for k-carrageenans.
Within the double-helical structure, the two chains are parallel, three-fold, right-handed are offset from the half-staggered position by 1.1 Å. and 28° about the common helix axis. Such an arrangement does not allow the occurrence of all the interchain hydrogen bonds.
Consistent with the differences in chemical composition, the conformational and packing features of i- and k-carrageenans are not identical. In the case of k-carrageenan, one can explain how the lack of sulfate group on every 3,6 anhydrogalactose residue may be reflected in the shorter helical pitch and in the offset positioning of the two chains.
The variations in molecular structure mirror the types of junction zones which are formed by these two polysaccharides and relate to the observed gelation properties. The gelation mechanism has been described in detail, and related to the solution behavior. The ionic selectivity observed for the double helix induction is also recognized for the gelation. The ion pairs which reduced the net charge of the single coil play a role in the double helix stabilization, as well as in the aggregation of double helix. In this process, the electrostatic repulsion is counterbalanced by cooperative hydrogen bond attractions. The mechanical properties of the gels formed in the presence of different counterions, follows the same order as for the stability of the double helices: potassium-k-carrageenan gels form at a lower polymer and ionic concentrations and have a higher elastic modulus and a higher melting temperature than tertramethylammonium-k-carrageenans gles which only form at much higher ionic concentrations and have a much lower modulus as shown by dynamic rheological experiments.








